Regulatory Affairs Services
With IRIS GLOBAL, turn the regulatory complexity of Latin America into a competitive advantage.
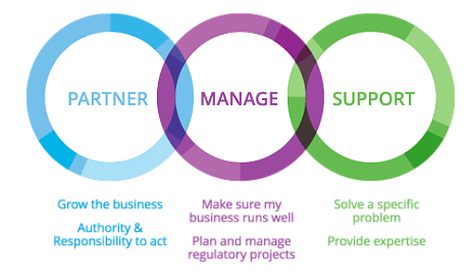
Our Regulatory Approach
IRIS GLOBAL works with its clients through various commercial management models. With an innovative “Globlocal®” strategy, IRIS GLOBAL acts as your primary regulatory affairs partner in a country or across a region.
IRIS GLOBAL's team of specialists in the region offers a comprehensive set of Regulatory Solutions, such as:
• Registration of products: with health authorities in LATAM (Pharmaceuticals, Nutritional Products, Cosmetics, Devices, etc.).
• Assessment of current capabilities and their fulfillment: Determine the performance status of regulations in one or more countries and identify the strengths, opportunities, weaknesses, and threats in the current regulatory approach.
• Development of regulatory strategies in Latin America: IRIS GLOBAL builds strategies, country by country, and integrates them in a cohesive and coherent manner for the region. The importance of a regional strategy is to optimize our clients' resources, since only a macro vision allows us to act proactively and find areas for improvement (for example, label optimization, development of a Master Dossier that minimizes the risk of observations by the authorities and, therefore, delays).

• Development of strategies for product submissionDevelopment of strategies for product submission: IRIS GLOBAL works with commercial and development teams to ensure that regulatory issues are proactively taken into account in product development.
• Gestión del ciclo de vida del producto: Prepare presentations and updates based on changes in the product lifecycle to ensure ongoing compliance and market access.
• Preparation and handling of dossiers prior to acceptance: IRIS GLOBAL prepares master dossiers, translates them into the local language, and submits them to the health authorities for approval.
•IRIS GLOBAL helps you stay ahead of the curve by learning about trends and regulatory changes before they happen.
During the project's development, the IRIS Team implements cloud-based tools and systems that guarantee a constant and secure flow of information and help visualize all project progress minute by minute.
Our services
Our latest content
Find out what's new at our company
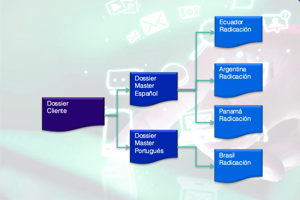
Master Dossiers
Iris specializes in preparing Complete Dossiers (Multi-countries) to ensure consistency, effectiveness, and follow-up throughout the product's life cycle.
Regulatory Intelligence
Regulatory intelligence is not information; it is experience with different regulatory entities.
Cloud Project Management
Iris manages secure cloud systems that provide visibility across all areas of the project.
Is there a service you can't find on our website?
Write to us. Our extensive experience gives us the tools to support you. It may simply not be listed.